Excess thermodynamic potential (excess Gibbs free
energy) of 1 mole of solution equals:

and after substitution of EHL activity coefficient expressions and transformation we obtain the following expression for thermodynamic potential of mixing of 1 mole of solution of B in A:
![Gm/(R·T) = GEm/(R·T)+xA·ln(xA)+xB·ln(xB) = xB·ln[xB/(xB+RBA·xA)]+RBA·xA·ln[RBA·xA/(xB+RBA·xA)]+(1-RBA)·xA·ln(1)+ln(QBA)·xB+[ln(KA)/RBA]·RBA·xA, where: Gm = thermodynamic potential of mixing of 1 mole of solution of B in A.](grafika/formula4.png)
The above expression may be obtained after assumption of a system composed of:
- mixture of solute and certain part of solvent, and
- remaining amount of pure (unaffected) solvent.
The two last components of this expression results from the difference between values of molar thermodynamic potential of both components in the solution and in a pure state (it is a consequence of establishing pure substance as standard state). I assume that structural changes (so also thermodynamic potential changes) apply to solvent connected with solute only. These changes are closely connected with behavior in high dilution range, when Henry Law is valid. The amount of solute is too small in this range, so it cannot influence solvent structure, and as a consequence solvent activity coefficient equals 1 (solute activity coefficient is constant). Some examples include methanol-water and ethanol- water systems (760 mm Hg), which meet Henry Law to alcohol mole fraction approximately equals 0,005 (Per Dalager, J. Chem. Eng. Data 14 (3), 298, 1969).
Below I give a schematic reciprocal of activity coefficient -mole fraction diagram in two-component system, congruent with EHL equations (Henry Law ranges are obviously too large).
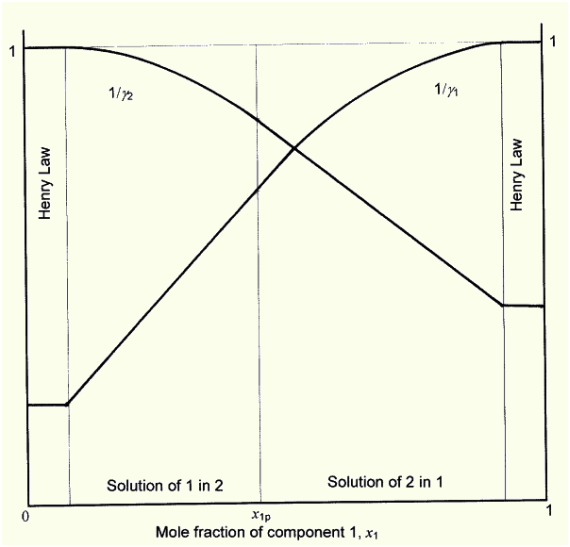
From the above considerations we may draw a conclusion, that, independent of the used equation, an exact description of activity coefficients in two-component system needs no less than four parameters: no less than two for a description of asymmetrical curve (in range out of Henry Law) and strictly two to take structural changes of mixture components into consideration.

and after substitution of EHL activity coefficient expressions and transformation we obtain the following expression for thermodynamic potential of mixing of 1 mole of solution of B in A:
![Gm/(R·T) = GEm/(R·T)+xA·ln(xA)+xB·ln(xB) = xB·ln[xB/(xB+RBA·xA)]+RBA·xA·ln[RBA·xA/(xB+RBA·xA)]+(1-RBA)·xA·ln(1)+ln(QBA)·xB+[ln(KA)/RBA]·RBA·xA, where: Gm = thermodynamic potential of mixing of 1 mole of solution of B in A.](grafika/formula4.png)
The above expression may be obtained after assumption of a system composed of:
- mixture of solute and certain part of solvent, and
- remaining amount of pure (unaffected) solvent.
The two last components of this expression results from the difference between values of molar thermodynamic potential of both components in the solution and in a pure state (it is a consequence of establishing pure substance as standard state). I assume that structural changes (so also thermodynamic potential changes) apply to solvent connected with solute only. These changes are closely connected with behavior in high dilution range, when Henry Law is valid. The amount of solute is too small in this range, so it cannot influence solvent structure, and as a consequence solvent activity coefficient equals 1 (solute activity coefficient is constant). Some examples include methanol-water and ethanol- water systems (760 mm Hg), which meet Henry Law to alcohol mole fraction approximately equals 0,005 (Per Dalager, J. Chem. Eng. Data 14 (3), 298, 1969).
Below I give a schematic reciprocal of activity coefficient -mole fraction diagram in two-component system, congruent with EHL equations (Henry Law ranges are obviously too large).

From the above considerations we may draw a conclusion, that, independent of the used equation, an exact description of activity coefficients in two-component system needs no less than four parameters: no less than two for a description of asymmetrical curve (in range out of Henry Law) and strictly two to take structural changes of mixture components into consideration.
- Next: Attempt to describe
Jump to: Top